Prediction of the effect of Cystathionine β-Synthase single amino acid mutations
Cystathionine β-Synthase (CBS) is a vitamin-dependent enzyme involved in cysteine biosynthesis via the transsulfuration pathway. The human CBS requires two cofactors for function, vitamin B6 (in the active form of pyridoxal 5’-phosphate [PLP], supplemented in the soluble form of pyridoxine) and heme (Skovby et al. 1984; Kery et al. 1994). Homocystinuria due to CBS deficiency (OMIM 236200) is a recessive inborn error of sulfur amino acid metabolism. Greater than 90 different disease-associated mutations have been identified in the CBS gene (Kraus et al. 1999). About one-half of homocystinuric patients respond to high doses of pyridoxine and several alleles are clearly pyridoxine remediable: A114V, R266K, R366H, K384E, L539S and the frequent I278T which accounts for 20% of all CBS mutant alleles.
The choice of a B6-dependent enzyme allowed us to test whether some mutations that reduce the function of an enzyme also exhibit cofactor remediation. The rationale for CBS as the target was several-fold: (1) the predictions of functional impact will be stronger if we start with an enzyme whose crystal structure is known, although in truncated form, (2) an assay for CBS activity and vitamin-responsiveness was feasible, (3) there is a vast body of literature surrounding CBS mutation and disease which indicates this enzyme is metabolically significant, and (4) because the enzyme is well-studied, there is much information to draw from, including clinically relevant, though rare, vitamin B6-remedial alleles.
In Vivo Yeast Complementation Assay
The scheme chosen to assay the human CBS mutants is a cell-based assay where the human CBS clone is expressed and functionally complements a yeast cell that has had the yeast gene for the orthologous enzyme, in this case CYS4, removed from the chromosome. This strategy has several desirable qualities; (1) expression of the human clone can be driven by a heterologous promoter and terminator so as to minimize transcriptional effects on protein levels, (2) the expression level of the complementing human clone can be modulated so as to restore growth but remain limiting, (3) defined growth media allows for the titration of exogenous B6 cofactor (in the form of pyridoxine), or by-pass of CBS function entirely by the addition of cysteine (in the form of glutathione).
A variant of this cell-based yeast growth assay was established in our laboratory and recapitulates earlier reports (Kruger and Cox 1994, 1995; Kim et al. 1997). It displays several of the important criteria mentioned above. The human gene was able to restore growth to cys4Δ cells in media lacking cysteine, but this growth was limiting and sensitive to CBS function (Figure 1A, note the growth phenotypes of three clinically relevant loss-of-function variants in relation to wild type). Critically, the growth of the CBS complementing strain was responsive to a range of concentrations of exogenous pyridoxine supplementation (Figure 1B). Qualitative assessments of CBS function can be made on solid media, while quantitative measurements of growth rate are determined by measuring cell density over time in liquid culture and calculating a slope of log-transformed data (Figure 1C). While it is difficult to quantitate specific activity of a given allele based on a growth assay, relative activities are obvious and this assay can be used to rank order activities as well as vitamin responsiveness. Finally, all clones were constructed with an antibody epitope tag (HA) at the 3’ end of the gene to allow facile protein detection.
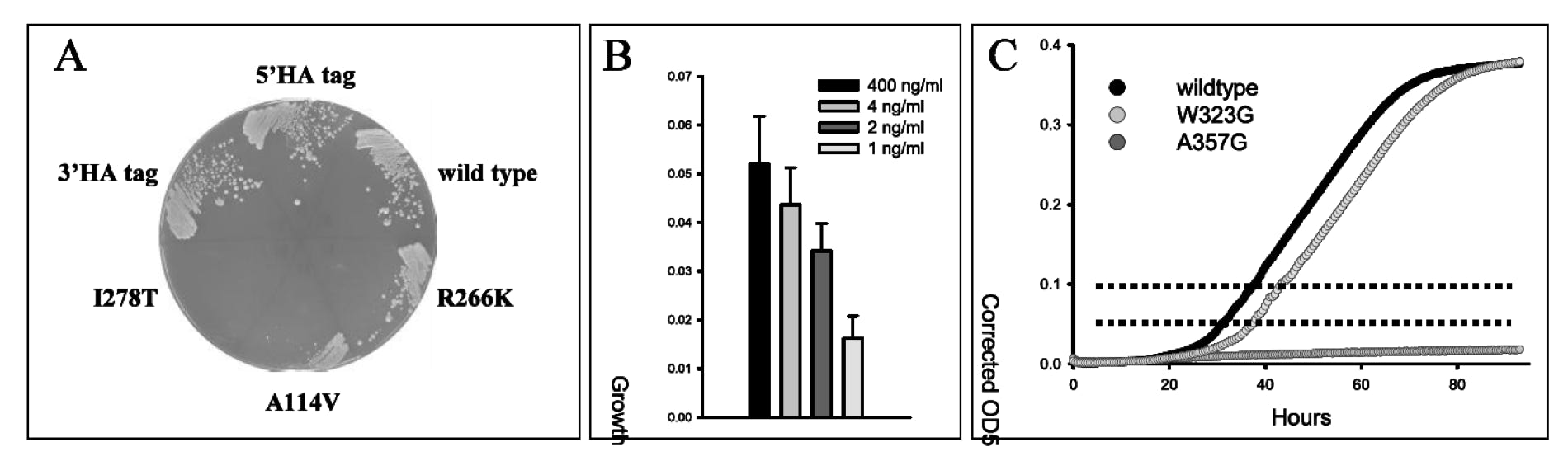
Figure 1. Yeast complementation assay for human CBS function. (A) Complementation of a yeast cys4Δ strain with expressed human CBS clones. Growth is dependent upon the level of mutant function. (B) Growth of the wild type hCBS complemented yeast strain is responsive to the level of exogenous pyridoxine (B6) supplementation. (C) Quantitative measurements of growth rates in liquid culture are determined between two fixed cell densities.
