Liver pyruvate kinase (L-PYK): predict the effects of missense mutations on kinase activity and allosteric regulation
Challenge: Pyruvate kinase
Dataset description: public
Variant data: registered users only, limited by CAGI Data Use Agreement
Last updated: 12 April 2016
This challenge closed at 9:00 PM PST (Pacific Standard Time) on 11 January 2015.
Download answer key, predictions, and assessment: registered users only, limited by CAGI Data Use Agreement. The answer key, predictions, and assessment files are accessible to registered users only, and their use is limited by the CAGI Data Use Agreement. Please log in access the file.
Presentations from the CAGI 4 conference: registered users only, limited by CAGI Data Use Agreement. Presentations are accessible to registered users only, and their use is limited by the CAGI Data Use Agreement. Please log in to access the file.
Summary
Pyruvate kinase catalyzes the last step in glycolysis and is regulated by allosteric effectors. Variants in the gene encoding the isozymes expressed in red blood cells and liver, including missense variants mapping near the effector binding sites, cause PK deficiency. A large set of single amino acid mutations in the liver enzyme has been assayed in E. coli extracts for the effect on allosteric regulation of enzyme activity. The challenge is to predict the impacts of mutations on enzyme activity and allosteric regulation.
Background
The Pyruvate kinase (PYK) catalyzes the last step in glycolysis, the transfer of phosphate from phosphoenolpyruvate (PEP) onto ADP to yield pyruvate and ATP. Mammals express four isozymes. The M1-PYK and M2-PYK isozymes are expressed from the same gene as a result of alternate exon usage. The isozymes expressed in red blood cells (R-PYK) and liver (L-PYK) are expressed from the same gene (pklr) as a result of alternate start site usage: R-PYK has an additional 31 N-terminal amino acids in comparison to L-PYK.
There are over 200 single nucleotide variants in the pklr gene associated with pyruvate kinase deficiency (PK deficiency [MIM 266200]), and ~160 of those cause amino acid substitutions in the protein (Warang et al., 2013; Pendergrass et al., 2006; Zanella et al., 2007). Altered glycolytic metabolism causes changes in steady-state concentrations of 2,3-bisphosphoglycerate, an allosteric effector of hemoglobin (Grace et al., 2015) and that altered regulation, in combination with reduced metabolism and therefore erythrocyte viability (hemolysis), results in anemia. Although the same mutations should alter L-PYK function, PK deficiency is considered a blood disease due to the health problems associated with anemia. Interestingly, PK deficiency provides resistance against malaria, similarly to sickle-cell anemia.
Approximately 50% of R-PYK expressed in E. coli is truncated. The same truncation does not occur when L-PYK is expressed in E. coli. However, the 31 amino acid difference between R-PYK and L-PYK appears to have little or no effect on enzyme function and regulation. Therefore, structure/function studies of enzyme and regulatory properties are common for L-PYK, but not for R-PYK. Understanding the allosteric regulation of L-PYK has its own appeal in attempts to develop allosteric drugs that activate R/L-PYK to counteract hyperglycemia.
The N-terminus of L-PYK is phosphorylated at Ser12 in response to hormone signals, and this phosphorylation reduces the affinity of the protein for substrate (Fenton, 2013; Fenton and Tang, 2009; Prasannan, Tang, and Fenton, 2012). Fructose 1,6 bisphosphate (Fru-1,6-BP), an intermediate in glycolysis, is a feed-forward allosteric activator of R/L-PYK. Alanine is an allosteric inhibitor of these isozymes: alanine is high during starvation when it acts both as a nitrogen carrier after amino acid metabolism in starving muscle and as a substrate for gluconeogenesis in the liver. The binding sites for both allosteric effectors are known. Human L-PYK has been co-crystalized with Fru-1,6-BP (Holyoak et al., 2013). The amino acid binding site is inferred from co-crystallization of other PYK isozymes with amino acids bound (Williams et al., 2006; Morgan et al., 2013).
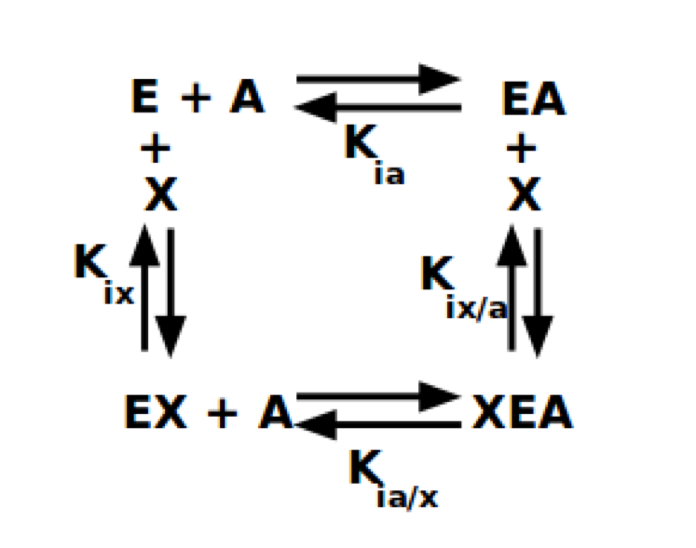
Several non-synonymous variants of R/L-PYK observed in PK deficiency patients fall in or near the allosteric effector binding sites. Therefore, modifications in allostery seem sufficient to cause disease. However, an experimental evaluation of allosteric regulation in a disease-related protein is dependent on the definition of that regulatory phenomenon. Even subtle differences in the definition of allostery influence experimental designs and interpretations. This challenge relies on defining allostery as an energetic energy cycle (Fenton, 2008; Reinhart, 2004) between substrate (A) and effector (X) binding to the enzyme (E). This energy cycle is represented in Reaction 1.
Reaction 1
In addition, to the dissociation constants defined in Reaction 1,
Qax= Kia/Kia/x = Kix/Kix/a.
Experiment
The dataset provided represents two experiments, both of which are characterizations of mutant human L-PYK proteins expressed in E. coli. The assays and data evaluations used to generate the dataset have been detailed previously (Fenton and Alontaga, 2009; Fenton and Hutchinson, 2009; Ishwar, Tang, and Fenton, 2015). Briefly, 12 concentrations of substrate were used to titrate activity as an evaluation of the apparent affinity of the enzyme for PEP. Note that PYK is thought to be a rapid equilibrium system. These titrations were repeated at 8 concentrations of effector. The extent that the apparent affinity for PEP shifts in the absence vs. saturating presence of effector is a measure of allostery. This shift is quantified as a ratio (Qax) of the apparent affinity in the absence vs. saturating presence of effector. (This evaluation also provides a value for the apparent affinity for substrate in the absence of effector and a binding constant for each of the two effectors in the absence of substrate.) Proteins used in these studies have been partially purified as NH4SO4 cuts and by extensive dialysis (Ishwar, Tang, and Fenton, 2015). However, because the proteins have not been purified to homogeneity, accurate protein concentrations, and therefore kcatvalues, are not available. Thus, in the challenge, we ask for prediction of kinase activity as present or absent, and not as a numerical value.
Experiment #1: When alanine is bound to L-PYK, the apparent affinity for the substrate PEP is reduced (i.e. allosteric inhibition). For each residue position with a side-chain that contacts bound alanine or is very near the bound alanine, site-directed mutations were created using degenerate codons/random substitutions. A total of 113 substitutions were introduced in 9 residue positions (one substitution per mutant protein). All mutant proteins were evaluated for presence/absence of enzyme activity. For the mutant proteins that retained enzyme activity, allosteric coupling (Qax) between PEP and alanine binding was evaluated. Allosteric coupling could not be measured for 12 mutants in the assay (see further discussion, below); these mutants are included in the dataset, but the corresponding predictions of allosteric coupling will not be assessed in the challenge.
Experiment #2: Alanine scanning mutagenesis was used to evaluate which non-alanine/non-glycine residues in L-PYK contribute to allosteric function. A total of 430 mutations were created. All mutant proteins were evaluated for presence/absence of enzyme activity. For the mutant proteins that retained activity, allosteric coupling (Qax) for inhibition by alanine and activation by Fru-1,6-BP were separately evaluated. Allosteric coupling of alanine could not be measured for 37 mutant proteins in this assay; allosteric coupling of F-1,6-BP could not be measured for 19 mutants in this assay; these mutants are included in the dataset, but the corresponding predictions of allosteric coupling will not be assessed in the challenge (see further discussion, below).
In both experiments, Qax = 1 indicates no allosteric effect (resulting either from absence of effector binding or absence of allosteric coupling), 0 < Qax < 1 indicates allosteric inhibition, and Qax > 1 indicates allosteric activation and has no theoretical upper bound. Also note that Qax is an equilibrium constant for a disproportionation equilibrium and, therefore, can easily be converted into free energy terms (Reinhart, 2004).
Note that determining allosteric effect requires measurement of enzyme activity and saturation of apparent affinity. We define three conditions under which allosteric effect cannot be determined: (1) Enzyme activity is undetectably low, so allosteric effect cannot be determined. (2) Enzyme activity is present but does not respond to effector, so allosteric effect cannot be determined. This may result from either failure of effector binding or failure of bound effector to alter PEP affinity. As previously noted, for the purposes of this challenge, we define the absence of an allosteric response as Qax = 1. (3) Enzyme activity is present but does not saturate under the assay conditions, so allosteric effect cannot be determined. This may result because a) substrate binding is so low even in the absence of inhibitor that it is not possible to simultaneously saturate both substrate and effector binding, b) same except inhibitor binding is so low, or c) there is such a large allosteric inhibition between the two ligands that it is impossible to simultaneously saturate. As noted above, mutations with assay results in this third category are present in the dataset, but the corresponding predictions of allosteric coupling will not be assessed in the challenge.
The evaluation of Qax also provides values for Ka (used in place of Kia since the PEP affinity is derived from initial velocity data instead of binding data) and Kix. We cannot provide the Ka and/or Kix values because that data would undermine the challenge to predict which mutant proteins retain activity. Nonetheless, the magnitudes of Ka and Kix would not provide clues to the magnitude of Qax due to definition given in the equation above.
Prediction challenge
Participants are asked to submit predictions on the effect of the mutations on L-PYK enzyme activity and allosteric regulation. Enzyme activity is a binary assay result: enter the probability that the mutant enzyme retains activity (0 = no activity detected, 1 = activity detected). Allosteric coupling is a continuous assay result: enter the predicted numeric value for Qax. For experiment #1, predict the results of the assay to measure coupling of the allosteric inhibitor alanine. For experiment #2, predict the results of the assay to measure coupling of the allosteric inhibitor alanine and the allosteric activator F-1,6-BP. Note that for allosteric activation by F-1,6-BP, Qax has no theoretical upper bound; the largest measured value in the experiment is 320. Each prediction must include a standard deviation; low SD indicates high confidence, and high SD indicates low confidence in the prediction. The predictions will be assessed against the results measured for each mutant protein in the assay.
Download dataset: This dataset file is available only to registered users. Please log in to access the file.
Download submission template: This submission template file is available only to registered users. Please log in to access the file.
Download submission validation script: This submission validation script is available only to registered users. Please log in to access the file.
Prediction submission format
The prediction submission is a tab-delimited text file. Organizers provide a file template, which must be used for submission. In addition, a validation script is provided, and predictors should check the correctness of the format before submitting their predictions.
In the submission template file, the two subsets of mutants in experiments #1 and #2 are indicated by header rows beginning with a “#”. These headers must be retained in the submitted file. Each data row in the submitted file must include the following columns:
- AA substitution - the mutation as listed in the dataset file.
- enzyme activity - the probability that the mutant enzyme retains activity (0 – 1); 0 = no activity detected, 1 = activity detected
- SD (enzyme activity) - the standard deviation of the prediction in column 2
- Qax-Ala - the predicted numeric value of allosteric coupling of alanine: 0 < Qax <= 1
- SD (Qax-Ala) - the standard deviation of the prediction in column 4
- Qax-F-1,6-BP - the predicted numeric value of allosteric coupling of F-1,6-BP: 1 <= Qax <= 320
- SD (Qax -F-1,6-BP) - the standard deviation of the prediction in column 6
- comment – optional brief comments on the basis of the predictions
In the template file, cells in columns 2-8 are marked with a "*". Submit your predictions by replacing the "*" with your value. No empty cells are allowed in the submission. For each experiment (#1, #2) and assay result (enzyme activity, Qax-Ala, Qax-F-1,6-BP), you must submit predictions and standard deviations for all or none of the mutations; if you are not confident in a prediction for a mutation, enter a large standard deviation for the prediction. Enter predictions for allosteric coupling even if you are confident that a mutant enzyme has no detectable kinase activity; mutant enzymes with no detectable activity will be excluded in the assessment of predictions of allosteric coupling. Optionally, enter brief comments on the basis of the predictions. If you do not enter predictions for an experiment or assay result, or if you do not enter a comment on a prediction, leave the "*" in those cells. For experiment #1, leave the "*" in columns 6 and 7, as allosteric coupling of F-1,6-BP was not measured. Please make sure you follow the submission guidelines strictly.
In addition, your submission should include a detailed description of the method used to make the predictions, similar to the style of the Methods section in a scientific article. This information will be submitted as a separate file.
To submit predictions, you need to create or be part of a CAGI User group. Submit your predictions by accessing the link:"All submission forms" from the front page of your group. For more details, please read the FAQ page.
Additional information
Structure of human L-PYK bound to Fru-1,6-BP: 4IMA and 4IP7 Structure of M1-PYK with amino acid inhibitor bound and used for location of amino acid binding site: 3N25, 2G50, and 4FXF.
References
Warang P, Kedar P, Ghosh K, Colah R. (2013).Molecular and clinical heterogeneity in pyruvate kinase deficiency in India. Blood Cells Mol Dis, 51, 133-7
Pendergrass DC, Williams R, Blair JB, Fenton AW. (2006).Mining for allosteric information: Natural mutations and positional sequence conservation in pyruvate kinase. IUBMB Life, 58, 31-8
Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. (2007).Pyruvate kinase deficiency: the genotype-phenotype association. Blood Rev, 21, 217-31
Grace RF, Zanella A, Neufeld EJ, Morton DH, Eber S, Yaish H, Glader B. (2015).Erythrocyte pyruvate kinase deficiency: 2015 Status report. Am J Hematol
Fenton AW. (2013).Are all regions of folded proteins that undergo ligand-dependent order-disorder transitions targets for allosteric peptide mimetics? Biopolymers, 100, 553-7
Fenton AW, Tang Q. (2009).An activating interaction between the unphosphorylated n-terminus of human liver pyruvate kinase and the main body of the protein is interrupted by phosphorylation. Biochemistry, 48, 3816-8
Prasannan CB, Tang Q, Fenton AW. (2012).Allosteric Regulation of Human Liver Pyruvate Kinase by Peptides that Mimic the Phosphorylated/Dephosphorylated N-Terminus. Methods Mol Biol, 796, 335-49
Holyoak T, Zhang B, Deng J, Tang Q, Prasannan CB, Fenton AW. (2013).Energetic coupling between an oxidizable cysteine and the phosphorylatable N-terminus of human liver pyruvate kinase. Biochemistry, 52, 466-76
Williams R, Holyoak T, McDonald G, Gui C, Fenton AW. (2006).Differentiating a Ligand's Chemical Requirements for Allosteric Interactions from Those for Protein Binding. Phenylalanine Inhibition of Pyruvate Kinase. Biochemistry, 45, 5421-9
Morgan HP, O'Reilly FJ, Wear MA, O'Neill JR, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD. (2013).M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci U S A, 110, 5881-6
Fenton AW. (2008).Allostery: an illustrated definition for the 'second secret of life'. Trends Biochem Sci, 33, 420-5
Reinhart GD. (2004).Quantitative analysis and interpretation of allosteric behavior. Methods Enzymol, 380, 187-203
Fenton AW, Alontaga AY. (2009).The impact of ions on allosteric functions in human liver pyruvate kinase. Methods Enzymol, 466, 83-107
Fenton AW, Hutchinson M. (2009).The pH dependence of the allosteric response of human liver pyruvate kinase to fructose-1,6-bisphosphate, ATP, and alanine. Arch Biochem Biophys, 484, 16-23
Ishwar A, Tang Q, Fenton AW. (2015).Distinguishing the interactions in the fructose 1,6-bisphosphate binding site of human liver pyruvate kinase that contribute to allostery. Biochemistry, 54, 1516-24
Data provided by
Aron Fenton, University of Kansas Medical Center
Revision history
11 Nov 2015 (v01): initial release
10 Mar 2016 (v02): answer key provided
18 Mar 2016 (v03): predictions provided
7 Apr 2016 (v11): conference presentations provided
12 Apr 2016 (v12): assessment provided